Increase efficiencies and productivity by automating core reporting tasks for medical writing.
Automate core regulatory report documents across the CTD pyramid and dramatically accelerate submission timelines.
Increase efficiencies and productivity by automating core reporting tasks for medical writing.
Automate core regulatory report documents across the CTD pyramid and dramatically accelerate submission timelines.
Standardize text outputs to ensure consistent, accurate, and compliant writing styles



1.
2.
3.
Discover how our AI-based, natural language generation (NLG) platform accelerates the time it takes for drugs to get to market and eliminates the risk of human error in the data review process.
Yseop Copilot’s automation packs automate the critical chain of preclinical documents to deliver a comprehensive level of report automation for highly accurate narratives.
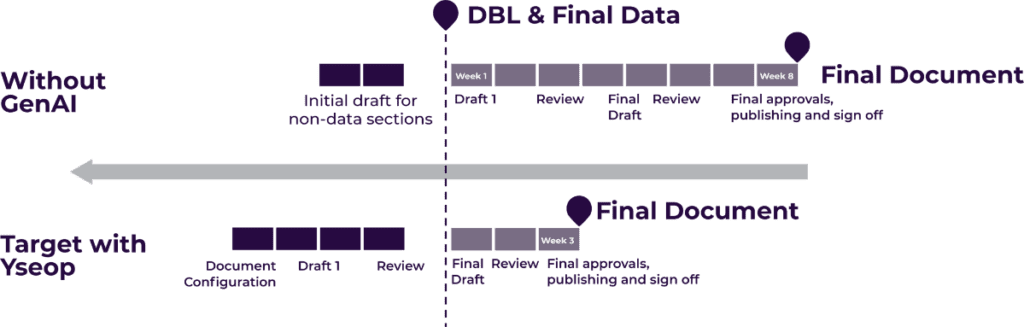
Let’s start automating your data to narratives today!