Increase efficiencies and productivity by automating core reporting tasks for medical writing.
Automate core regulatory report documents across the CTD pyramid and dramatically accelerate submission timelines.
Increase efficiencies and productivity by automating core reporting tasks for medical writing.
Automate core regulatory report documents across the CTD pyramid and dramatically accelerate submission timelines.

Yseop Copilot is the most advanced regulatory writing platform, streamlining everything from narrative generation to final submission. Built for scale, Copilot supports the world’s top life sciences teams.
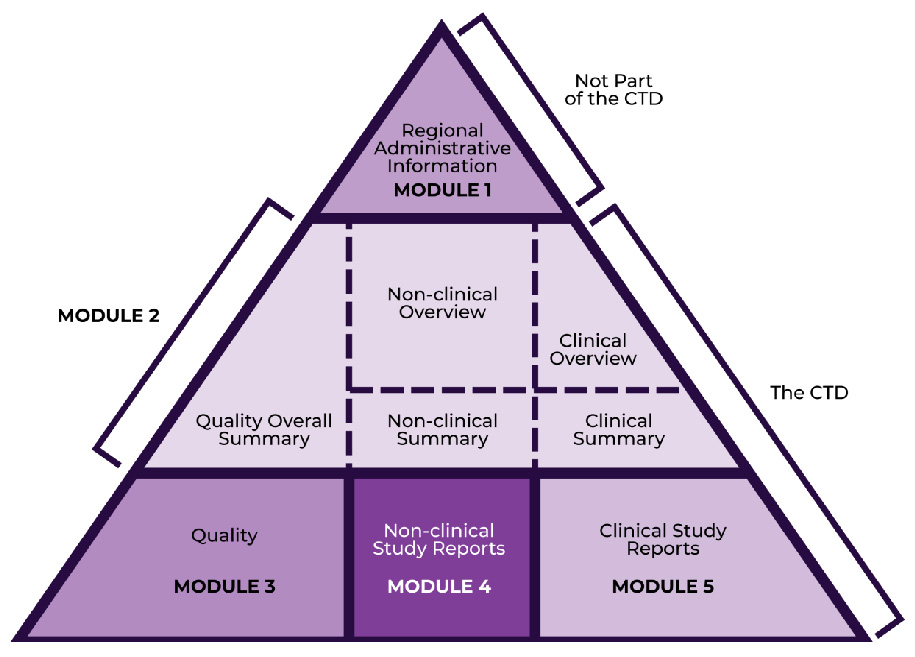
Regulatory Document Coverage Across eCTD Modules



1.
Create fully formatted documents from structured and narrative sources
Draft directly in Microsoft Word and Veeva Vault
2.
Lock sections, reuse content, and validate against source changes
Automate updates across documents, templates, and regions
3.
Built-in QC, audit trails, and formatting
Assemble full dossiers for compliant eCTD delivery

With one-click draft generation directly in Microsoft Word, regulatory writers work in familiar environments while scaling their document production. Yseop Copilot helps life sciences teams improve report quality, retain talent, and meet deadlines with confidence.
Documents that match customer templates and submission standards


Yseop Copilot integrates seamlessly with the platforms your teams already use, enabling a fully connected and compliant regulatory workflow—from kickoff to final submission.











Let’s start automating your data to narratives today!